With the rapid spread of the new coronary pneumonia epidemic, Europe has become a severely affected area. In the process of preventing and controlling the epidemic, due to the serious shortage of medical masks, protective clothing and other medical supplies, Europe has released a huge demand for protective equipment. For the EU market, CE certification is essential. A variety of medical CE certificates have appeared on the market, which is dazzling. There have also been frequent news that manufacturers’ mask masks have been detained and the CE certificate is reported to be invalid as a false report. Let’s talk about the reasons:
Why is your mask detained?
Based on the information currently collected by ZRLK, the mask was detained for several reasons:
Incomplete qualifications
Export masks should include these qualifications:
1. Epidemic materials managed by ordinary masks and other non-medical devices can be directly exported by manufacturers or foreign trade companies with import and export rights.
2. Medical masks and other epidemic-related materials, if the production enterprise has the right to import and export, they can directly export; foreign trade companies need to add sales of medical devices within the scope of business, and record the second-class medical devices. The medical device related qualification certificates and test reports of the listed manufacturers.
Most countries and regions have certification or registration requirements for imported masks and other medical devices, such as Korean KF certification, Japanese PDMA certification, US FDA certification, EU CE certification, etc. Domestic manufacturers need to be certified in advance according to the relevant requirements of the country where the foreign customer is located .
If you export to the EU without a CE certificate, it must be detained by the customs in accordance with fakes and inferior goods.
Certificate fraud
For mask export, there are people who dare to PS certificate, specifically deceived the merchants who are not clear about the mask market.
Recently, a foreign trade company undertook the export of masks once, because the market for masks was not familiar, and the docking factory provided PS fake certificates, and they did not understand. As a result, the goods were detained at customs.
The certificate is true, but not recognized by the EU
The certificate is really issued from a certain certification company, but the certificate issued by this certification company is not recognized by the European Union at all, so it is impossible for the customs to release it.


(The content of the PPE suspicious certificate published on the official website of the European Security Union)
On April 7, the European Safety Federation (European Safety Federation) published an article on its official website, notifying a group of certification companies that issued suspicious certificates. Many CE certifications were issued by small companies, which cannot be regarded as formal The "CE certification" is invalid.
PPE suspicious certificate original content view address: https://eu-esf.org/covid-19/4513-covid-19-suspicious-certificates-for-ppe
The "CE" mark in the EU market is a mandatory certification mark. Whether it is a product produced by an enterprise within the EU or a product produced by another country, if it is to be circulated freely in the EU market, it must be affixed with the "CE" mark to indicate that the product It meets the basic requirements of the EU "Technical Coordination and Standardization New Method" directive. This is a mandatory requirement imposed on products by EU law.
According to EU regulations, all products exported to the EU need to obtain CE certification, and the CE mark is affixed to enter the European market.
What kind of CE certificate is formal?
Query the official website of the notified body
Generally large EU certification authorities will open a window for querying certificates on their official website. After logging in to the agency's official website, there will be a page for querying certificates. You can enter the manufacturer's English name, certificate number and other information to check whether there is a matching CE certificate. If it does, it may be a real certificate.
Of course, this method is only applicable to the case where the issuing authority just provides the query service. For institutions that have not opened the certificate query service, it will not work. So for such cases, when we get a medical CE certificate, how should we identify whether it is issued by an EU certification authority?
We can still try to start with the issuer of this certificate in your hand, check the EU official website to see if it has the corresponding certification qualifications of the European Union Medical Device Directive MDD 93/42/EEC or MDR Medical Device Regulation (EU) 2017/745 .
1. The official website of the European Union MDD 93/42/EEC Medical Devices Directive:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=13
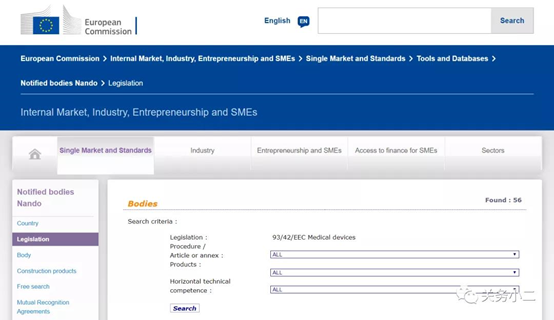

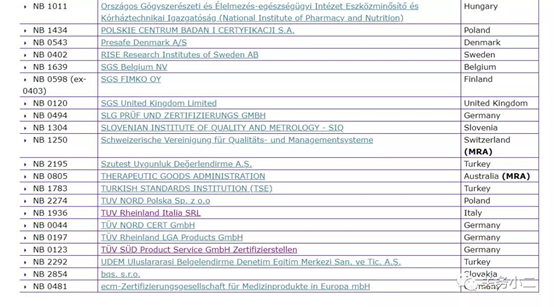
(List of 56 MDD authorized institutions)
Through the official website of the European Union, we can see that there are 56 notified bodies authorized by the MDD 93/42/EEC Medical Device Directive. The detailed list of institutions, the bulletin number, and the scope of their qualified products are listed in detail above.
2. From May 26, 2020, the MDR (EU) 2017/745 medical device regulation will officially replace the current MDD medical device directive in the EU. It can also be found on the official website of the EU. Currently, the notified bodies with MDR authorization are currently only 12 companies.
EU official website MDR (EU) 2017/745 medical device regulations authorized institutions to query the address:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=34

(List of 12 MDR authorized institutions)
Therefore, if your medical CE certificate issuing agency is not within the scope of the above list, it means that it does not have the EU certification for medical products, let alone the issuance of CE certificates, then, unfortunately, you The "CE certificate" obtained is invalid.
In addition, the process of CE certification of medical device products can also be analyzed to complete the identification.
CE certification process analysis and identification
Taking a mask as an example, first, confirm whether the mask belongs to a medical device. Masks are divided into medical masks and protective masks. Exports to Europe and America must comply with the relevant EU regulations:
Personal protective masks:
The regulation is EU2016/425 (PPE);
The standard is EN149.
Medical masks:
The regulations are 93/42/EEC (MDD) or EU2017/745 (MDR);
The standard is EN14683.
1. Personal protective masks Personal protective masks are not medical devices and do not need to meet the requirements of EU medical regulations. You can complete CE certification in accordance with the PPE Personal Protection Directive. There are 112 notified bodies authorized by the PPE Personal Protection Directive on the EU official website.
The official website of the European Union (EU) 2016/425 Personal Protective Equipment Authorized Inquiry Address:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=155501
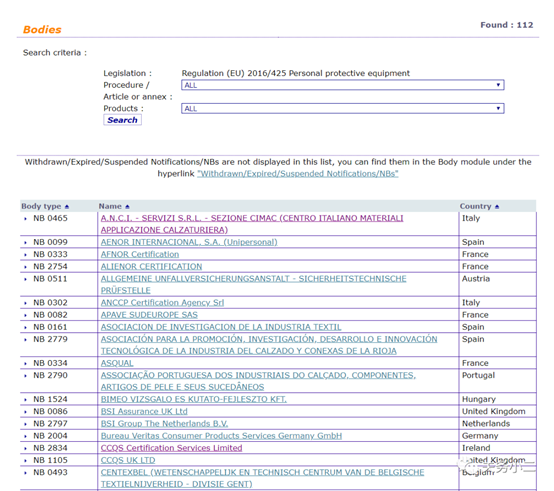



(List of 112 PPE authorized institutions)
CE certificate identification (PPE):

(Source: CCQS certification)
2. Medical mask
If it is a medical mask, you need to complete the certification according to the medical device regulations. For medical masks, it is necessary to further confirm whether it is sterile.
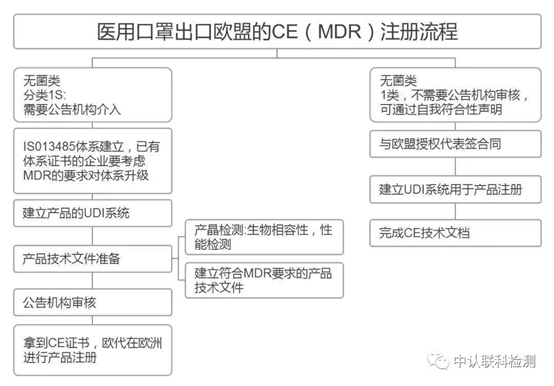
If it is a sterile medical mask, it belongs to a class of medical products with sterilization in the European Union. It must be CE-certified in accordance with the Medical Device Directive/Regulation MDD/MDR. In such cases, an authorized notified body must be involved.
If it is a non-sterile medical mask, it is CE self-declared according to the medical device directive/regulation MDD/MDR. The enterprise does not need to pass the certification of the notified body. After preparing the relevant documents and test reports, it can complete the declaration of conformity. .
As far as the current situation is concerned, in view of the difficulty and long time required for CE certification of sterile medical masks, most manufacturers have chosen non-sterile medical masks to produce and complete certification.
A key point needs to be drawn here. Since it is the manufacturer that declares CE compliance, how can it be announced that the agency issues the CE certificate? If CE certificates cannot be issued, what are the so-called certificates obtained by many enterprises? Let's find some templates to see:

Please carefully study the contents of the certificate: "Verification of the presence of the Technical Files in regards of the Medical Devices Directive..." means that this certificate proves that the organization has reviewed that the company has prepared technical documents in accordance with the medical device regulations.
"This document has been issued on voluntary basis and not as NB..." means that this certificate was issued voluntarily and does not mean that I executed the matter in the name of the notified body. "…Declares that the only scope of the assessment is to verify the existence of the declaration issued by the manufacturer or an applicant under its own responsibilities" means that this certificate only verifies the manufacturer or applicant from their perspective based on their own The declaration of conformity issued by the responsibility exists.
Therefore, this kind of so-called certificate is not a CE certificate issued by an authorized notified body in the true sense. So please be sure to open your eyes to identify the authenticity, and don’t be exploited by speculators.
Other questions about the CE certificate
Which specific countries in the EU need CE certification?
EU countries: Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden, Estonia, Latvia, Lithuania, Poland, Czech Republic, Slovakia, Hungary, Slovenia, Malta, Cyprus , Bulgaria, Romania.
Although the following countries are not in the EU, they still need the CE mark: the three members of the European Free Trade Association EFTA: Iceland, Liechtenstein, Norway. Semi-EU countries: Turkey.
How much does it cost to make a CE certificate for masks?
Between 10,000 and 20,000, depending on the product, the certification price of medical masks and ordinary masks are different.
How long does it take to make a CE certificate for masks?
About 7 to 12 days, the time of each certified agency is different, they will be divided into first-hand agents and second-hand agents.
How long can a CE certificate last?
The validity period of the product CE certificate applied by the enterprise is generally about 5 years, but the specific certificate validity period depends on the specific circumstances of the EU CE certification regulations. The CE certification itself has no validity period. If there are no new standards issued by the regulations, and the manufacturer, supplier, production process, etc. of the product have not changed, then the CE certification is always valid. However, if the implemented standards or instructions are revised, upgraded, updated, etc., the product may need to be re-evaluated, difference tests are added, the certificate is replaced, and even a new application may be required.
What is the process of applying for CE certification?
(1) Application for certification-the enterprise provides technical information of the product, and the certification body determines the applicable instructions and standards based on the information;
(2) Sign the contract-the enterprise and the certification body sign the CE mark certification contract;
(3) Product pre-inspection-the certification body will send the samples to the authorized relevant laboratory for testing;
(4) Final product inspection-the enterprise adjusts the product, completes the relevant procedures, and the laboratory gives the final inspection report;
(5) Enterprise rectification-the enterprise will make rectification according to the final inspection report;
(6) Technical review-laboratory conducts technical review of products;
(7) Certificate issuance-The product is qualified for evaluation, and the notified body authorized by the European Union issues a certificate to the product.
ZRLK provides customers with personal protective masks, N95 masks and other CE certification, FDA certification, professional engineers to answer your questions, if you have any questions or are unclear, please contact our company. Enterprises in need can directly contact ZRLK to consult related businesses, and seize the opportunity to pass as soon as possible.













